
Microsatellite Instability Assay
Microsatellite Instability (MSI) assay utilizing multiplex, fluorescent PCR and capillary electrophoresis to enable sensitive detection of five mononucleotide repeat markers
Microsatellite Instability Assay
Microsatellite Instability (MSI) assay utilizing multiplex, fluorescent PCR and capillary electrophoresis to enable sensitive detection of five mononucleotide repeat markers

Clinical Genomics Laboratory Services
Next Generation Sequencing Services
Microsatellite Instability Assay
Immune Landscape Signatures
Oncomine Precision Assay
Tumor Mutational Burden
TruSight Oncology 500 (TSO500)
TruSight Oncology 500 ctDNA
Whole Exome Sequencing
Liquid Biopsy ctDNA
TruSight Oncology 500 ctDNA
Single Cell and Spatial Genomics
Single Cell RNA Sequencing
Spatial Genomics
Biomarker Discovery
Technology Platforms
Bioinformatics Services
Ordering and Client Login
Central Laboratory Services
Microsatellite instability (MSI) results from an accumulation of insertion or deletion of repeating units during DNA replication in tumor cells with a deficient mismatch repair (MMR) system. MSI status is associated with response/resistance to certain immune checkpoint inhibitors. Q2 Solutions MSI assay utilizes multiplex, fluorescent PCR and capillary electrophoresis to enable sensitive detection of five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) in tumor FFPE specimens. Microsatellite instability at two or more mononucleotide loci is interpreted as MSI-High; microsatellite instability at a single mononucleotide locus is interpreted as MSI-Low; no instability at any of the loci tested is interpreted as microsatellite stable (MSS).
Example of Colon Cancer FFPE Tissue Specimen Demonstrating MSI-High Status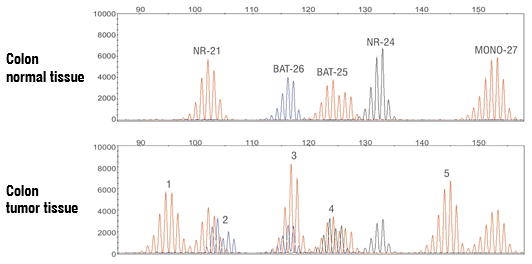
Example capillary electropherogram demonstrating reference, normal profile in normal tissue (upper panel) and MSI-High profile in tumor FFPE tissue (lower panel). New MSI events labeled 1−5 are detected in tumor tissue.
| MSI Assay Specifications | |
|---|---|
| Markers | BAT-25, BAT-26, MONO-27, NR-21, and NR-24 microsatellite loci |
| Specimen Requirements | Tumor: 3 x 5um FFPE tumor slides or 3 curls or FFPE block or DNA. Minimum 20% neoplastic cellularity required. Normal: either whole blood (1-3 mL, K2EDTA) or tumor FFPE slides with clearly indicated normal tissue area or 3 x 5um normal tissue FFPE slides, curls or block. If H&E stained slide with indicated tumor normal areas is not available, will perform H&E assessment and determine specimen adequacy. |
| Assay Method | OncoMate® MSI Dx Analysis System |
| System Compatibility | Thermo Fisher Scientific SeqStudio Flex |
| Regulatory Tier | RUO, GCP, CAP-CLIA |
| Deliverables | MSI Status report. Exact number of MSI markers available as custom report. |
Related Services & Solutions
Related Thought Leaders Insights
Liver Disease Capabilities
As a leading laboratory services organization for trials across the globe, we integrate therapeutic insights, state-of-the-art technologies, best-in-class methods and quality systems to optimize your...
Visium Spatial Profiling
Visium spatial profiling is a next-generation sequencing method that enables the analysis of cellular relationships by combining transcriptomics with histological techniques. It has advantages over...
Special Immunoassays Test Menu
This brochure highlights the specialty immunoassays performed by Rules-Based Medicine, a Q2 Solutions company. RBM provides an extensive menu of quantitative protein biomarkers, including multiplexed...








