
TruSight Oncology 500 ctDNA
(TSO500 ctDNA)
Minimally invasive, pan-cancer next-generation sequencing assay for biomarker identification and comprehensive genomic profiling across 523 genes
TruSight Oncology 500 ctDNA
(TSO500 ctDNA)
Minimally invasive, pan-cancer next-generation sequencing assay for biomarker identification and comprehensive genomic profiling across 523 genes

Clinical Genomics Laboratory Services
Next Generation Sequencing Services
Microsatellite Instability Assay
Immune Landscape Signatures
Oncomine Precision Assay
Tumor Mutational Burden
TruSight Oncology 500 (TSO500)
TruSight Oncology 500 ctDNA
Whole Exome Sequencing
Liquid Biopsy ctDNA
TruSight Oncology 500 ctDNA
Single Cell and Spatial Genomics
Single Cell RNA Sequencing
Spatial Genomics
Biomarker Discovery
Technology Platforms
Bioinformatics Services
Ordering and Client Login
Central Laboratory Services
A Hybrid-Capture Approach for Target Enrichment of 523 Genes Across Various Tumor Types
Gain an expansive view of your liquid biopsy samples by targeting 523 cancer-related genes using the TruSight Oncology 500 ctDNA assay. Not only does this assay identify all relevant variants (DNA fusions, SNVs, CNVs, insertions, and deletions) across various tumor types, but it also reports tumor mutational burden (TMB) and microsatellite instability (MSI) scores.
Our comprehensive genomic panel has advantages over individual biomarker assays in its ability to detect multiple biomarkers with one test, saving time, money, and samples. Q2 Solutions can meet your profiling needs by leveraging trusted technology from Illumina® in combination with our global laboratory network and expert support team.
An Efficient, Reliable Solution for Non-Invasive Tumor Characterization
 Minimal input, extensive results
Minimal input, extensive results
Obtain full exon coverage of 522 out of 523 genes using as little as 3 mL of plasma, 20-30 ng of cfDNA, or 10 mL of whole blood. TSO500 ctDNA can detect biomarkers without the need of a matched normal sample.
 Simple, cost-effective workflow
Simple, cost-effective workflow
Detect multiple biomarkers with a single assay to save time, money, and samples. The distributable kit enables global implementation.
 Confident variant detection
Confident variant detection
Obtain a high-resolution view of variants enabled by TSO500 ctDNA’s hybrid-capture chemistry and unique molecular indices (UMIs).
 Measurement of key immuno-oncology biomarkers
Measurement of key immuno-oncology biomarkers
Gain insight into TMB score and MSI score in addition to variant calls. TruSight Oncology 500 ctDNA can reproducibly assess TMB.
 Expansive services enabled by our global network
Expansive services enabled by our global network
Our centralized, scientific operational oversight combined with our global laboratory footprint enable comprehensive, large-scale trial support. We enable clinical development support irrespective of geography.
 Flexible customization to satisfy specific needs
Flexible customization to satisfy specific needs
We work with you to understand your specific needs, and our team provides cost-effective recommendations from custom assay development to specialized bioinformatics support.
TSO500 ctDNA Variant Detection
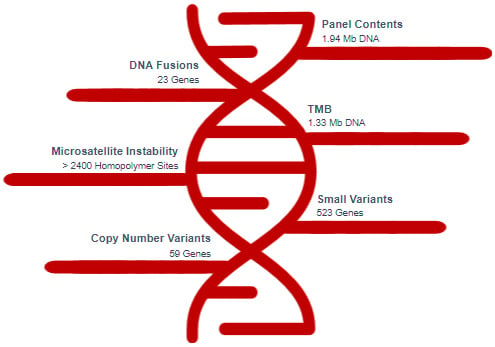
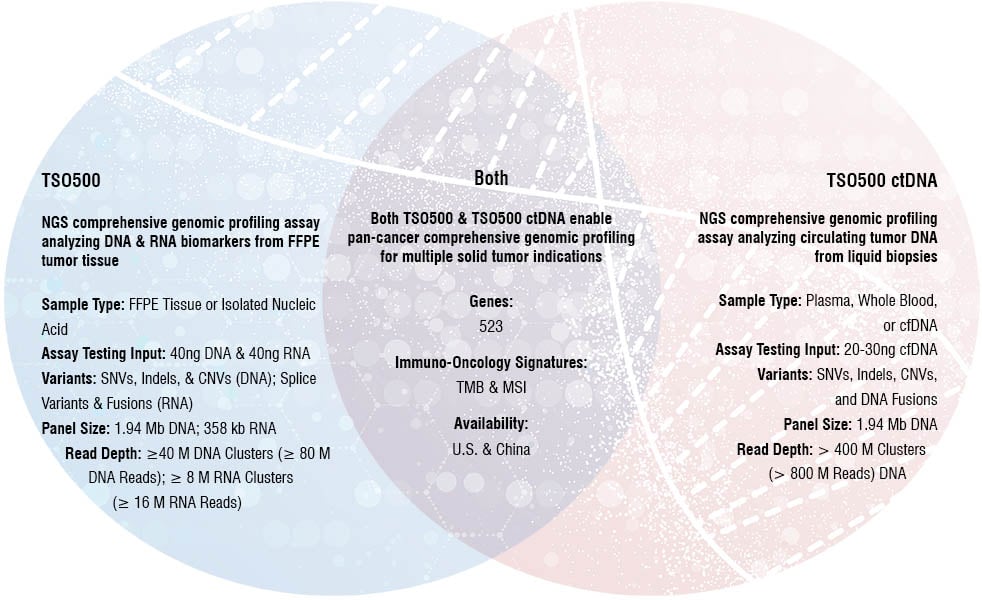
TSO500 Assay Comparison
Deep Sequencing Enabled by Q2 Solutions’ Subject Matter Expertise
Feel confident in your results with the trusted Illumina® software pipeline. Our software teams validate the most up-to-date software to ensure you are receiving optimal results. Q2 Solutions can develop custom reporting and filtering to meet your specific research needs. Q2 Solutions was one of the earliest adopters of DRAGEN™. Our technical support teams help you understand your deep sequencing data with our superior bioinformatics knowledge.
Deliverables
Q2 Solutions provides a text-delimited, combined variant output file for all biomarkers
- QC Metrics
- CNV Outputs
- DNA Fusion Report
- Genomic VCFs (SNVs & Indels)
- Variant Call Annotations
- MSI Scores
- TMB Scores
- FASTQs Upon Request
- BAMs Upon Request
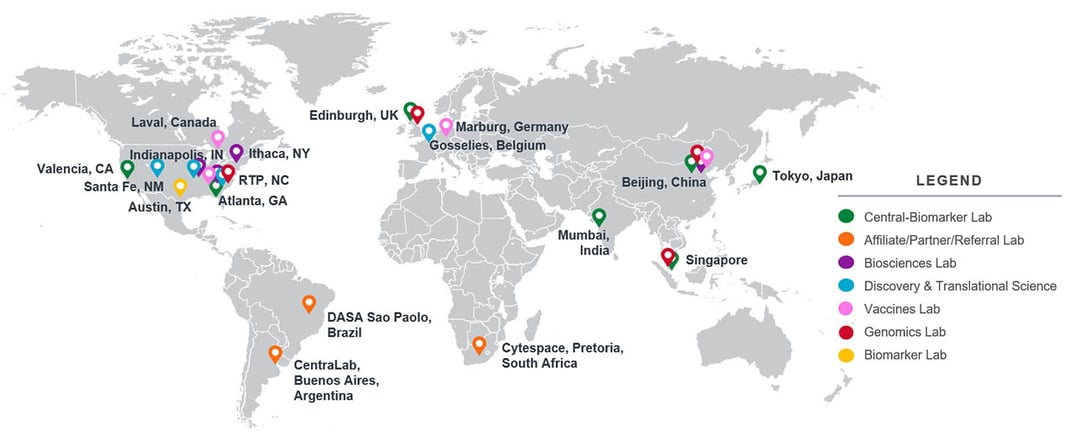
Supporting Studies Around the World
Our centralized, scientific, and operational oversight combined with our global laboratory footprint enables comprehensive, large-scale trial support. You will be supported every step of the way by our scientific project management team who will work with you to understand the most cost-effective recommendations to meet your specific needs. We are committed to transparency so you can feel confident in our promises of quality and timely delivery. We can expert support team can meet your research needs from nearly anywhere in the world with our genomic centers in the United States, China, United Kingdom, and Singapore.
Learn More About TSO500 with Q2 Solutions
Posters
- TMB Standardization by Alignment to Reference Standards
- Panel-based TMB Analysis of Matched Tumor and Plasma Specimens Using Illumina’s TSO500 NGS Assay
- Comprehensive Genomic Profiling in FFPE Tissue Sample using TruSight Oncology 500 Assay
Presentations
- Approaches to Demonstrating Commutability of Various NGS Reference Standards for Targeted ctDNA Analysis
- Analytical Validation of Illumina’s TSO500 ctDNA Assay
Whitepaper
Fact Sheets
Webinars
- Bridging the Gap in Liquid Biopsies with CTCs and ctDNA
- Comprehensive Genomic Profiling from Liquid Biopsy Samples: Clinical Trial Considerations
Podcasts
- The Standardization of TMB by Alignment to Reference Standards
- Liquid Biopsy Collection in Clinical Development Programs
Press Release
Videos
Simplify Your Work

Request a Quote
Request a quote today to discover how TSO500 with Q2 Solutions can advance your research.
Ship Samples
Ship your samples to one of our global genomic laboratory centers.
Get Results
Obtain in-depth data revealing insights into your solid tumor samples.
Related Services & Solutions
Related Thought Leaders Insights
Non-invasive Detection of Immunotherapy Biomarkers using TruSight Oncology 500 (TSO500)
The TruSight Oncology 500 (TSO500) ctDNA assay employs a hybrid capture-based approach targeting 523 clinically relevant genes and leverages unique molecular indices to enable ultra-low frequency...
Minimal Residual Disease Detection: Key Considerations for Clinical Development in Oncology
Measurement of minimal residual disease (MRD) is one of the best predictors of treatment outcome for leukemia, lymphoma and myeloma.Two approaches of specific interest due to the innovation they...
Gene expression for keeping pace with immuno-oncology breakthroughs and biomarker identification
Author: Victor Weigman, Ph.D., Associate Director, Associate Director, Translational Genomics, Q2 SolutionsThe excitement surrounding immuno-oncology (I-O) is being driven by results seen in the...








