services
Clinical Genomics Laboratory Services
We offer a range of genomic laboratory services to support drug discovery, precision medicine and clinical development. Our industry-leading team advances research by delivering scientific insights that help clients maximize the value of their data.
Welcome to IQVIA Laboratories Genomics, where we specialize in providing state-of-the-art genomics laboratory services. Our team of experts utilizes the latest technology and techniques to offer comprehensive genomic testing and analysis. From genetic sequencing to personalized medicine, we are dedicated to advancing the field of genomics.
of Genomic Data in 2024
Extensive experience working with the top 10 pharmaceutical companies.
IQVIA Genomics has partnered with >125 leading/top biotech companies.
Since 2001, we have been a recognized industry leader in genomic testing, setting the standard for quality and innovation.
Genomics Laboratory Services
Explore our state-of-the-art genomics laboratory, offering comprehensive services in oncology genomics, clinical genomics analysis, and more.
View our services, capabilities, and locations here.
Anatomic pathology
- Fresh tissue and FFPE capabilities
- H&E creation/assessment and digital slide imaging available
- Pathologists onsite
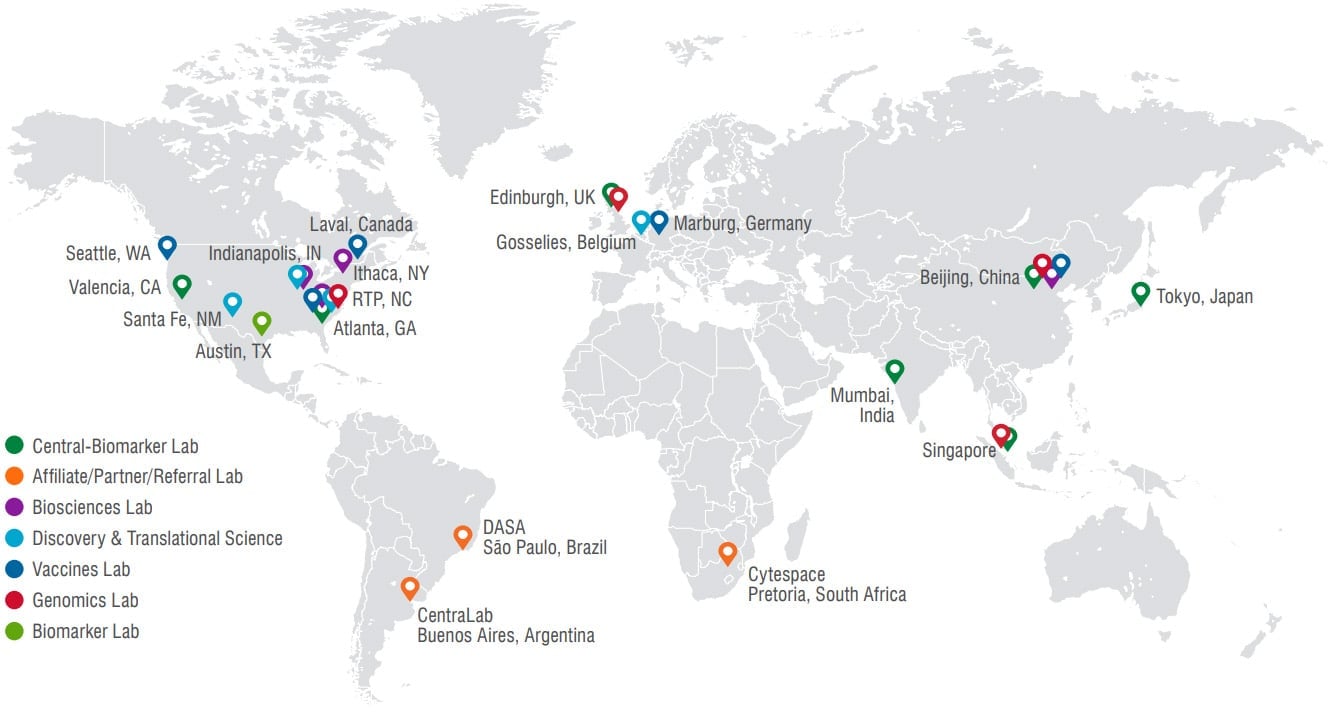
- Located in USA, China, UK, and Singapore.
Isolations
- DNA and RNA extraction capabilities from a large spectrum of original material, including FFPE, blood, tissue, cells, plasma, and saliva
- Location availability in USA, China, UK, and Singapore.
Whole human genome sequencing
- Optimized method for FFPE and intact material, using low input and minimal amplification
- Illumina NovaSeq system available
- Location availability in USA, China, and UK.
DNA exome sequencing
- Low input procedure optimized for intact and FFPE material
- Flexible hybridization method that allows for use of various standard and customized ROI probe sets
- Illumina NovaSeq system available
- Location availability in USA, China, UK, and Singapore.
RNA sequencing
- Globin removal method to remove globin mRNA from blood
- Library preparation from both intact and degraded RNA from FFPE, blood, tissue, and cells
- Hybridization-based method targeting the human transcriptome
Poly-A selection method for intact material - Ribosomal RNA depletion method for degraded and non-coding RNA
- Illumina NovaSeq system available
- Location availability in USA, China, and UK.
Targeted sequencing
- Library preparations from RNA/DNA from FFPE, blood, tissues, cells, and plasma
- Hybridization-based methods targeting different panels of interest
- NovaSeq system
- Location availability in USA, China, and UK.
Thermo Fisher sequencing
- Manual and Genexus library preparation with various panels
- Customizable with DNA, RNA, or simultaneous profiling available
- Genexus, Ion Chef, S5 XL and PGM Dx platforms available
- Location availability in USA, China, UK, and Singapore.
Single cell RNA sequencing
- Simultaneous analysis of the transcriptome and cell surface proteome
- Various assays including immune repertoire, surface protein profiling, and single-nucleus
- 10X Chromium and Illumina NovaSeq systems available
- Location availability in USA
Spatial RNA and protein profiling
- Customizable, spatially restricted hybridization- and antibody-based RNA and protein quantification, respectively
- Immunofluorescence-guided cell selection and stratification performed by qualified pathologists
- Targeted NanoString nCounter or whole transcriptome (i.e., 18,000+ protein-coding genes) Illumina next-generation sequencing readouts available
- Location availability in USA
PCR assays
- Customizable probe amplification assays for different targets of interest
- Multiple platforms for expression profiling, genotyping, and copy number analysis
- ddPCR and qPCR systems available
- Fluidigm system available at USA site
- Location availability in USA, China
NanoString nCounter panels
- Probe Hybridization based capture, RNA copies counted by nCounter
- No amplification required
- Customizable
- Up to 800 targets in a single reaction
- Location availability in USA, China
Bioinformatics
- A range of analyses to support all service lines
- Custom analysis and algorithm development including biostatistics support
- Standard pipeline analysis available globally (including China)
- Location availability in USA (Centralized in RTP location)
Regulatory standards
Our Quality Management System (QMS) is based upon the standards set forth in Good Clinical Practices (GCP), College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA).

CAP
CAP Accreditation since 2017
CLIA (USA)
CLIA Certified since 2010
CLIA (Edinburgh)
CLIA certified since January 2024
GCP
IQVIA QMS is compliant with the applicable Good Clinical Practices for clinical and genomic testing conducted
NYS (USA only)
NYS certified since 2018
Genomics Laboratory can help
Our global capabilities support patient needs by delivering 1.2 Petabytes of Genomic Data in 2024. We provide both standardized and customized solutions, backed by over 20 years of expertise, 50+ CDx projects, and industry recognition since 2001. IQVIA Labs Genomics has extensive experience working with the top 10 pharmaceutical companies and has partnered with over 125 leading biotech companies.