Blog
Clinical trial sample and consent tracking in the Digital Age
Moving from pen and paper to automated tech
Breakthroughs in medicine and technology have amplified the need for clinical trial sponsors and partners to move from pen-and-paper or Excel spreadsheet tracking to automated systems for clinical trial sample and consent tracking. They face new complexities in sample management at every step, and delays pervade a highly regulated industry that is procedure-driven and not generally associated with terms like agility and flexibility.
The distributed ecosystem of clinical studies includes central labs, sponsors, clinical research organizations (CROs), lab testing and biobanking. Challenges compound when a sponsor or CRO leads multiple studies.
Patient sample consents help researchers determine which samples they can use and for what purpose, but tracking can be costly when not approached with a comprehensive, modern solution. Tracking consent using state-of-the-art technology offers a more efficient process than older methods.
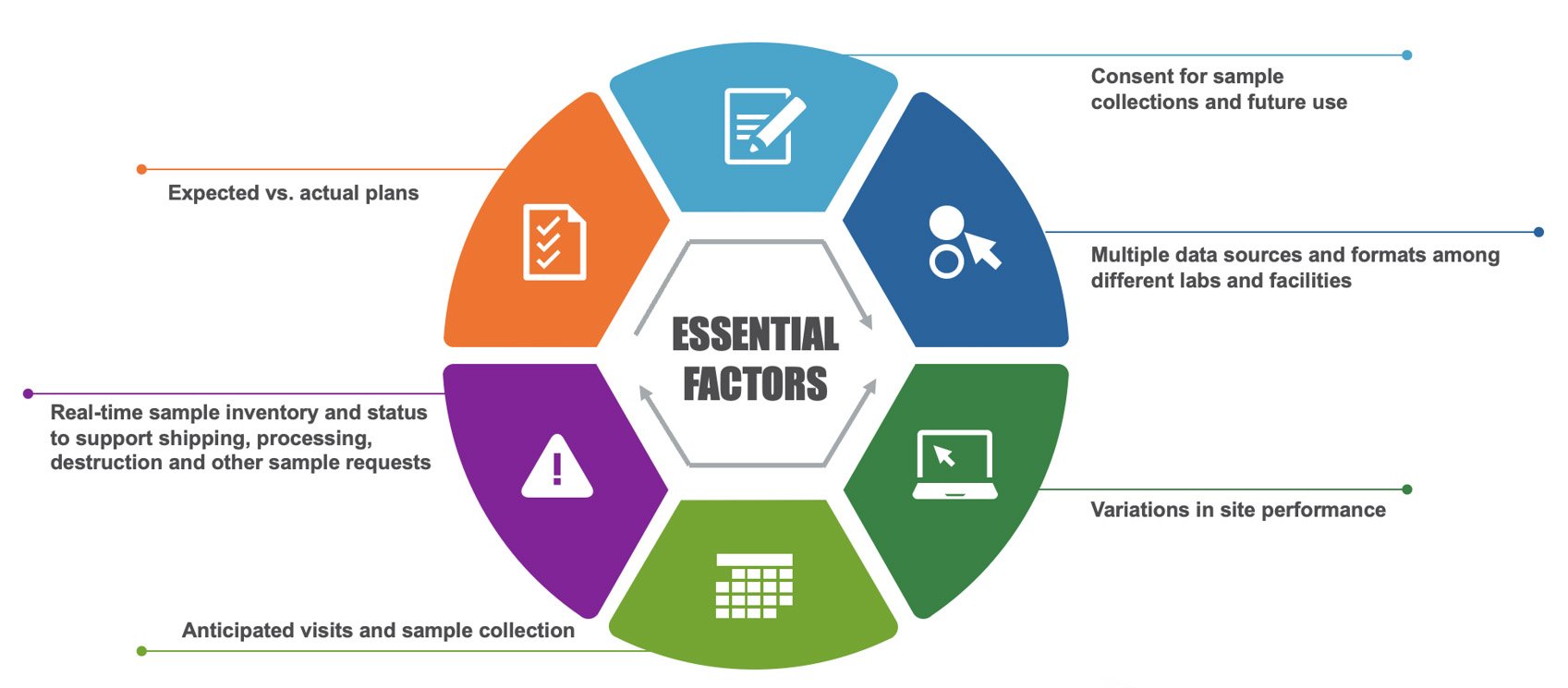
Large and small biotech companies benefit from clinical trial sample and consent management practices. Yet many still operate using antiquated techniques, such as spreadsheets or pen and paper, according to an Industry Standard Reports-issued report, “Sample and Consent Tracking Qualitative Interviews,” commissioned by Q2 Solutions.
Take the data gleaned from the report issued in September 2022. Nearly 73 percent of respondents reported using Excel spreadsheets as their primary sample and consent tracking method. Still, they identified significant disadvantages of this automated technology.
- Dependence on manual entry and prone to errors
- Data not available in real-time
- Time-consuming to manage
- Not a scalable solution; it can only be used in smaller studies
Another 27 percent reported using pen-and-paper logs. The advantages of using this simple method are that it’s accessible to all, has low starting costs, and is easy to implement. But much like using spreadsheets, pen-and-paper tracking is a labor-intensive, inefficient way to track these vital components of clinical trials, leading to unnecessary risks for biobanks.
Older, manual methods are prone to human error, necessitate additional resources for reconciliation and tracking, and are potentially duplicative.
Next-generation biobanking and the future of clinical trials
BioFortis, a Q2 Solutions company, offers a more holistic option than previously available for clinical trial sponsors and CROs. Labmatrix® Clinical Trial Sample and Consent Tracking (CTST) is a configurable and upgradable system that provides comprehensive sample lifecycle support for in-study sample and sample consent management and tracking. This includes future-use virtual or physical biorepositories, also known as next-generation biobanking, and associated data mining.
With Labmatrix®, researchers can build customizable dashboards with data-intensive visuals. Illustrations help tell a clinical trial's story, including the research plan's specifics and what samples can be used and when. The dashboards also help manage risk in a sophisticated ecosystem in which central labs, third-party labs and electronic data capture (EDC) software all provide data at different stages of the research.
Labmatrix® report families capture critical narratives that illustrate the flow of study resources. With the upgrade to Labmatrix® 10, research teams can reap the benefits of upgraded dashboard capabilities with customizable dashboards, Qiagram® queries as data sources and an export-import function for consistency across environments.
How can I have full visibility into my clinical trial samples?
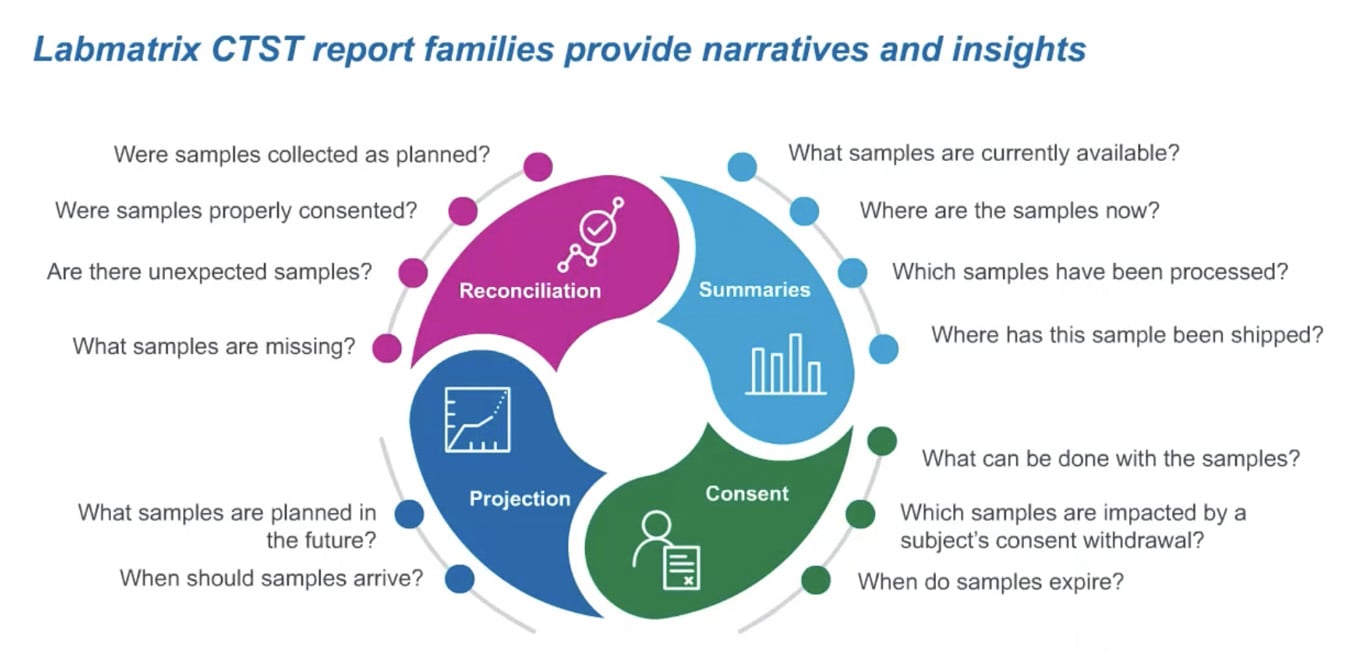
Q2 Solutions understands that sponsors must have complete visibility into each sample in a clinical trial as it moves from patient to repository throughout the distributed ecosystem. They must account for every sample at any point in the trial. Labmatrix® enables sponsors to have complete visibility of their data.
As a data hub, Labmatrix® helps the flow of information. It is not a system of record or an authoritative source of the data; instead, it helps study teams detect issues in the data from different vendors, which can then be queried and corrected in source systems. Data is loaded on a daily or weekly basis through a secure FTP, so there is no need for manual data entry into the software.
Summary reports are also a valuable tool within Labmatrix®. These provide details about study samples, including current status and location, what samples have completed testing, and shipping and receiving details. Additionally, users can easily compare expected plans to what actually occurred at each step and consent for sample collection and allowable use, answering questions such as:
- Have subjects withdrawn consent?
- Has consent expired (based on a predetermined retention period)?
- What upcoming visits and collections are scheduled?
Reconciliation reports highlight how all sample subject data comes together using EDC data and sample data from vendors:
- Were samples collected according to the plan?
- Was consent obtained?
- Are there any missing or unexpected samples that should be investigated?
Together, these reports offer a complete view of all sample data within the Labmatrix® system to provide insights on sample quality that users can address in near-real-time.
Making data smarter in small and worldwide clinical trials
Information hubs are a central component of big data to work with massive datasets requiring scalable solutions to store and analyze data efficiently. Qiagram® is a unique tool for researchers to make data useful by making it more intelligent. It enables customization without coding and empowers researchers by providing an environment for deep partnership designed to deliver a scientific intelligence tool that addresses many challenges researchers face in the data exploration phase.
The tool offers easy access to dynamic data. Users can query across multiple data domains in a simple, secure interface. All data can be audited to ensure compliance with regulatory guidelines.
Collaboration is another essential feature of Labmatrix®. Users can share queries with any researchers and informaticians involved in a study, offering unprecedented transparency of the research group’s thought processes. Security access is extended for each user, ensuring only those who need access to the data can view it.
Labmatrix® is compatible with Microsoft SQL Server, Oracle, Netezza and Synapsee.
Labmatrix® software: A better solution for sample and consent tracking
A biobank is a liability, not an asset — unless and until it can provide significant value for scientific research. BioFortis sees the biobank as just one component in an integrated research ecosystem. With next-generation biobanking, you can reap the benefits of a well-annotated patient and sample database that supports:
- Precision medicine
- Clinical trials
- Translational research
- Patient registries
Labmatrix® powers centralized management so research teams can reduce delays, costs and regulatory risks for clinical trial samples and manage and document business needs for future research use of clinical biospecimens. Q2 Solutions collaborates with CROs to develop site sample collection capabilities using the unique CTST software.
A partnership with Q2 Solutions helps researchers do more with their available resources to reduce operational trial costs, non-compliance risks, regulatory findings, and costs associated with inefficiencies inherent in the status quo. The cutting-edge software reduces effort, cost and risk in a way that’s not available anywhere else.
To learn more, visit BioFortis at Booth No. 913 at the 2023 SCOPE Summit for Clinical Ops Executives. Don’t miss our informational session at 12:30 p.m. Tuesday, Feb. 7. BioFortis Vice President and General Manager David Kaye will present “Clinical Trial & Consent Management: Too Important to Leave to Chance.”









