
Infectious Diseases
Assays Contributing to Infectious Disease Therapies
Infectious Diseases
Assays Contributing to Infectious Disease Therapies

Recent events force the question: What is the best plan to reduce the burden of infectious disease globally through vaccination and prevention? Q2 Solutions has long been at the forefront, aiding in the development of new vaccines faster and with better methodologies in partnership with premier organizations focused on this task. We continue to commit to prioritizing a swift response to global threats to public health through innovation, collaboration, and action.
- Our partner, the Coalition for Epidemic Preparedness Innovations (CEPI), has established a global network of laboratories transferring viral functional methods and reagents worldwide for rapid vaccine development. Their goal is to reduce the threat of future outbreaks and enable equitable access to lifesaving vaccines.
- Another partner, the Bill & Melinda Gates Foundation, also aims to reduce health inequities through new tools and strategies that reduce infectious diseases, such as HIV, malaria, and pneumonia. In addition, they invest in deep technical expertise and novel platforms for vaccine development to accelerate innovation for better, faster, and affordable vaccines.
- To ensure the limited resources for infectious disease research are put to best use, the WHO lists diseases that currently pose the greatest public health risk, such as SARS-CoV-2 (part of our collaboration efforts), Crimean-Congo hemorrhagic fever, Ebola virus disease, and Lassa fever.
- Reducing food-borne bacterial infections is an urgent concern across the globe. Targeting specific serotypes of pathogens such as Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin-producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia is one way to better understand outbreaks and prevention.
Custom Assay Development Services Expedite Your Research
Our longstanding expertise and track record in infectious disease crosses the spectrum of assay development and testing.
- Early Development - We have a highly experienced team and exceptional in vitro bioassay development support for candidate selection. Evaluate immune response with custom, high-performance functional and immunogenicity assays utilizing human immune cells. With efficient, top-tier technology platforms, we will help you ensure functionality and improve your program’s chance of success.
- Preclinical - The right model can make all the difference. We have developed numerous in vivo models for specific disease states and our experts tailor models to suit your objective.
- Expanded Clinical Phase Services - Whether you need validated assays for vaccines targeting viral or bacterial agents, or large and small molecule bioanalytical services, such as LC-MS, cell-based, or ELISA assays (and multiplex platforms like MSD and ELISpot), at Q2 Solutions, we have the scalable solution.
- Protein Science - Q2 Solutions scientists are specialized in molecular biology, protein expression, protein purification, and protein characterization. We tailor our programs to suit your needs, providing well-characterized products of the highest quality. Our experts will help you choose the most beneficial environment in which to produce your molecules, such as human cell lines for viral protein production. Furthermore, to expedite development and ensure supply, we produce our own critical reagents (antigens) for in-house and external clients.
- Vaccine - Agnostic of modality, the nature of the infectious agent drives our assay development and selection utilizing a wide range of solutions. We have the flexibility to adapt assays to study not only the initial disease, but also any variants that may develop.
- Global Footprint - Q2 Solutions is strategically expanding to provide you with more options, expertise, and services. Partnership with UK Health Security Agency expands our capacity and global reach. Investments such as the January 2021 acquisition of GSK’s GCLP-certified clinical bioanalytical laboratory in Marburg, Germany add capacity and expertise. Ongoing projects in Marburg include development of meningitis functional assays and in collaboration with our Laval site, a study of influenza.
Assays for Vaccine Development and Testing
At Q2 Solutions, we approach each vaccine program with disciplined agility: scientific excellence and full transparency with the flexibility, ingenuity, and capacity for the quick decision-making needed. Our expertise is built on a foundation of 20 years of experience in vaccine efficacy and safety evaluation as a partner to industry leading vaccine manufacturers worldwide. Cutting-edge technology platforms and state-of-the-art laboratories enable us to best develop, qualify, and validate single and multiplex immunological assays and sample testing to support vaccine development.
Q2 Solutions has seven integrated platforms that help you progress from vaccine development through clinical evaluation. Our main focus is on the evaluation of the efficacy of the vaccine —be it in the developmental stages or in clinical immunological responses. Critical reagents and well-established assays are indispensable to the efficient assessment of vaccine candidates. We take pride in our ability to develop innovative and critical reagents in-house. This capacity allows us to scale rapidly and helps control batch-to-batch variations of assay materials— crucial factors in maintaining operational excellence, high quality data, and world class science for our clients.
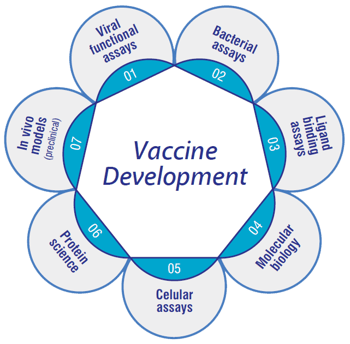 Integrated Vaccine Platforms
Integrated Vaccine Platforms
Challenges to develop suitable assays and tests increase when vaccine development is accelerated in response to emerging infectious disease threats. At Q2 Solutions, we develop custom assays relevant to the disease state which is further adapted as the disease evolves. This progressive approach provides an early prediction of how the vaccine translates later phase clinical trials. A successful vaccine project requires not only an experienced partner who understands the complexity presented in the research, but one that also has the capability to scale operations.
Our end-to-end translational support for your vaccine and immunotherapy programs ranges from lead compound selection to post-market registration. In addition, our innovative and fit-for-purpose working models are ideal for evaluating the efficacy and immunogenicity of your vaccine candidates needed for regulatory approvals.
- Plaque Reduction Neutralization Tests (PRNT) and microPRNT – Neutralizing antibodies promote the elimination of pathogenic viruses from the body and play a vital role in antiviral response. The gold standard for determining antibody efficacy is virus neutralization. The ability to rapidly monitor the neutralizing response is crucial for the development of efficient vaccines and drugs. The microPRNT assay can be utilized for high-throughput and small volume assay conditions. Results from this assay quantifies the number of Plaque Forming Units (PFU), which measures the reduction of PFU compared to each well report out the level of neutralization.
- Neutralization and Microneutralization – The principle of the neutralization assay is the same as the PRNT. The readout of this test does not count the Plaque Forming Unit (PFU) as the endpoint, instead, the level of infectivity is indirectly measured by the survival of cells or utilizing reporter genes in virus such as luciferase or GFP.
- Psuedo-particle Neutralization (PNA) – Pseudo-particles are replication-deficient viruses. These viruses can be modified to harbor the key glycoprotein from highly infectious virus and a reporter (luciferase or fluorescent protein) that can be used in the assay instead of the live virus. Infectivity of the virus can be quantified based on the reporter readout. PNA assays can be performed with lower biocontainment (Biosafety Level 2 facilities) and are a safer alternative to using live infective pathogens. Neutralizing activities are measured by the level of reporter gene expressed (luciferase or GFP) in multi-well plate readers that improves accuracy and objectivity as compared to manual plaque counting.
- Hemagglutination Inhibition (HAI) – Many viruses such as influenza viruses are capable of inducing hemagglutination. HAI test is useful to assess functional antibody induction following an infection or vaccination. Measurement of antibody response pre- and post-vaccination is required to understand vaccine-induced immunity.
Our functional antibody assays for bacteria include complex assays such as:
- Serum Bactericidal (SBA) – SBA assays are used to measure the level of antibody in the serum required to kill a bacterium through complement activation.
- Opsonophagocytic (OPA) – OPA assays quantify the amount of serum antibody required to opsonize bacteria and undergo phagocytosis in the presence of complement proteins. The proportion of phagocytic cells that engulf the bacteria is measured using automated image analysis systems.
- ELISA – Enables 384 or 96-well colorimetric-mediated readouts to detect and quantify peptides, proteins, and antibodies.
- AlphaLISA – This is a no-wash alternative to ELISA that is designed to detect a variety of samples using a simple all-in-one-well protocol. This assay requires significantly lower sample volumes (about 5 µL of sample per well) as compared to a conventional ELISA. AlphaLISA reduces hands-on steps and total assay times than ELISAs. AlphaLISA assays offer economies that increase throughput and easily automate without loss of sensitivity.
- Meso Scale Discovery (MSD) – For highly sensitive detection of specific binding, SULFO-TAG™ antibodies are electrochemiluminescence (ECL) based system in 96-well plates. SULFO-TAG™ illuminates upon stimulation with an electrical current, allowing for amplification of signals, allowing the user to validate bindings using low concentrations of the substrate, thus allowing the user to do larger screens.
- Luminex – Luminex uses a bead-based approach to assays. This allows them to create multiplexed assays, with beads recognizing different targets. A simple streptavidin-biotin system is then used to detect captured analytes with biotinylated antibodies. Using this analyte agonistic technology allows the Luminex platform to characterize antigens with minor interference from other antigens. In turn, the cauterization of the immune response for each antigen is more accurate, ultimately allowing the user to determine the identity of neutralizing antibodies facilitating protective immunity. This combination of ease of use and more accurate antigen screening is important when developing new vaccines dominated by combination and multivalent vaccine development platforms.
The molecular biology platform performs PCR-based assays to support vaccine studies. Our qPCR and RT-qPCR assays are used for quantification of the vaccine candidate particles (genome copies) in the samples to support viral shedding studies.
- qPCR
- RT-qPCR
Vaccine development and evaluations require highly sensitive and quantitative methods that can measure a broad range of molecules such as cytokines, antigenic and cellular markers. Our cellular assays include the following:
ELISpot – Enzyme-linked immunosorbent spot (ELISpot) is a highly flexible and versatile assay that can be adapted to multiple readout formats. ELISpot assays are quantitative and measure key cellular functions of immune system cells. They are useful for assessing both adaptive (humoral) and innate (cell-mediated) immune responses to a vaccine.
- Humoral Responses (B-Cell)
- Detection of Antibody Secreting Cells (ASC) – IgG/IgA
- Detection of memory B-cells
- Cell-Mediated Immune Responses (T-Cell)
- Cytokine quantification
- Vaccine efficacy (IFN-γ)
- Quantify immune responses (Th1/Th2)
- Perforin & Granzyme B
- Cytotoxic T-cell responses
In order to identify mediators of short- and/or long-term immunity, a robust method of identification, sorting, and characterization is needed.
Related Services & Solutions








