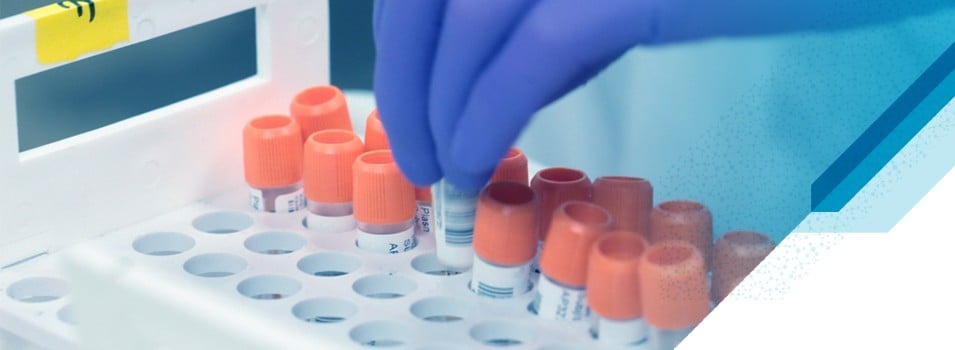
Labmatrix® Clinical Trial Sample & Consent Tracking

Labmatrix® Clinical Trial Sample & Consent Tracking
Qiagram® Data Exploration
Next Generation Biobanking
How We Can Work Together
BioFortis Resources
BioFortis User Forum
BioFortis Sales and Support
For biomarker-driven clinical trials, patient samples are as important as patients themselves; critical decisions are dependent on sample analyses. However, current support for sample operations does not scale well with the increased volume and complexity of these trials. This reduces study team productivity, delays trial execution, and poses significant regulatory compliance risks.
Labmatrix® is a technology-enabled solution for clinical trial sample and consent tracking. Utilized in 1000+ trials, it empowers study teams to monitor the health of clinical trials from a sample-centric perspective across the distributed ecosystem of sites, labs, vendors, and biobanks —whether you are currently insourcing, outsourcing (e.g. to CROs), or partially outsourcing your sample tracking and management endeavors.
Solution Features
- Automated reconciliation of planned vs. actual biospecimen collection and shipment
- Up-to-date, virtual biospecimen tracking across network of clinical trial sites, partners and vendors
- Notification on logistics details, resolution of operational issues for trial biospecimens
- 100% user-configurable search and report on ALL data points
- Scalable data standardization and integration across multiple sources – without programming
- Computable patient consent, improving in-trial and future-use sample utilization
Solution Benefits
- Reduce the risk of sample logistics becoming the bottleneck in clinical trial execution
- Acquire actionable insights into the health of clinical trial operations from a sample-centric perspective
- Enable study team to discover biospecimen problems earlier, and resolve issues more effectively
- Ensure regulatory compliance with patient informed consent regarding sample retention, use, and destruction
- Extend utilization of banked samples beyond the current study
- Reduce sample storage costs and optimize storage capacity
Prior to your study's regulatory submission, instead of having to wait for the usual 1 - 3 months period after database lock due to sample reconciliation efforts, Labmatrix® enables your team to reconcile samples on an ongoing basis, minimizing the lag time attributed to sample verification.
Finally, you can apply the same level of rigor in managing clinical trial samples as you do in managing the patients themselves.
Related Services & Solutions









